Home | Category: Physical Oceanography
CHEMISTRY OF THE OCEAN
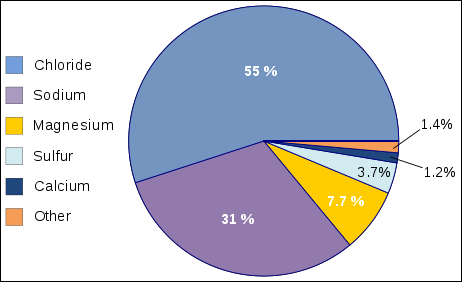
Composition of ocean water Major Constituents of Sea Water (Ions): A) Chlorine: 55.3 percent; B) Sodium: 30.8 percent; C) Sulfate: 7.7 percent; D) Magnesium: 3.7 percent; E) Calcium: 1.2 percent; F) Potassium: 1.1 percent.
Major Constituents of Sea Water (Atoms): A) Chlorine: 55.3 percent; B) Sodium: 30.8 percent; C) Magnesium: 3.7 percent; D) Sulfur: 2.6 percent; E) Calcium: 1.2 percent; F) Potassium: 1.1 percent.
Chemical oceanography is the study of the chemistry of Earth's oceans. An interdisciplinary field, it involves the distributions and reactions of both naturally occurring and anthropogenic chemicals from molecular to global scales. Due to the interrelatedness of the ocean, chemical oceanographers frequently work on problems relevant to physical oceanography, geology and geochemistry, biology and biochemistry, and atmospheric science. Many chemical oceanographers investigate biogeochemical cycles, and the marine carbon cycle in particular attracts significant interest due to its role in carbon sequestration and ocean acidification. Other major topics of interest include analytical chemistry of the oceans, marine pollution, and anthropogenic climate change. [Source: Wikipedia]
Colored dissolved organic matter (CDOM) make up an estimated 20 to 70 percent of carbon content of the oceans, with higher amounts near river outlets and lower amounts in the open ocean. In terms of biochemistry, marine life is similar to terrestrial life except that they inhabit a saline environment. One consequence of their adaptation is that marine organisms are the most prolific source of halogenated organic compounds ( substances that contain carbon and hydrogen, but where one or more hydrogen atoms have been replaced by a halogen – chlorine, bromine, fluorine or iodine).
Related Articles:
OCEANOGRAPHY AND STUDYING THE SEA ioa.factsanddetails.com
PHYSICS OF THE OCEAN: PRESSURE, SOUND AND LIGHT ioa.factsanddetails.com
TEMPERATURE IN THE OCEAN ioa.factsanddetails.com
DEVICES, TECHNOLOGY AND MEASUREMENTS USED IN OCEANOGRAPHY ioa.factsanddetails.com
Websites and Resources: National Oceanic and Atmospheric Administration (NOAA) noaa.gov; “Introduction to Physical Oceanography” by Robert Stewart , Texas A&M University, 2008 uv.es/hegigui/Kasper ; Woods Hole Oceanographic Institute whoi.edu ; Cousteau Society cousteau.org ; Monterey Bay Aquarium montereybayaquarium.org
RECOMMENDED BOOKS:
“Chemical Oceanography and the Marine Carbon Cycle” by Steven Emerson and John Hedges) Amazon.com
“Seawater: Its Composition, Properties and Behavior” by Open University (1995) Pergamon Press Amazon.com
“The Blue Machine: How the Ocean Works” by Helen Czerski, explains how the ocean influences our world and how it functions. Amazon.com
“How the Ocean Works: An Introduction to Oceanography” by Mark Denny (2008) Amazon.com
“The Science of the Ocean: The Secrets of the Seas Revealed” by DK (2020) Amazon.com
“Atmospheric and Oceanic Fluid Dynamics: Fundamentals and Large-Scale Circulation” by Geoffrey K. Vallis (2006) Amazon.com
“Descriptive Physical Oceanography” by Lynne Talley (2017) Amazon.com
“Essentials of Oceanography” by Alam Trujillo and Harold Thurman Amazon.com
“The Unnatural History of the Sea” by Callum Roberts (Island Press (2009) Amazon.com
“Ocean: The World's Last Wilderness Revealed” by Robert Dinwiddie , Philip Eales, et al. (2008) Amazon.com
“An Introduction to the World's Oceans” by Keith A. Sverdrup (1984) Amazon.com
“Blue Hope: Exploring and Caring for Earth's Magnificent Ocean” by Sylvia Earle (2014) Amazon.com
“National Geographic Ocean: A Global Odyssey” by Sylvia Earle (2021) Amazon.com
Saltwater and Salinity
Salt water is more dense than fresh water and supports a fish’s body better. Sea water is about 3.5 percent salt. Though the amount of salt in the world's oceans remains mostly unchanged, the brine concentration in the topmost layer varies around the globe.
Salinity refers to the concentration of salt and other inorganic compounds in seawater. Salinity is one of the most basic measurements used by ocean scientists. When combined with temperature data, salinity measurements can be used to determine seawater density, which is a primary driving force for major ocean currents. [Source: NOAA]
At its simplest, salinity is the total mass of dissolved material, in grams, contained in one kilogram of seawater. Because it is defined as a ratio, salinity is dimensionless—it has no units. Natural variations in dissolved salts are extremely small, so salinity must be defined and measured with great precision. Across most of the world’s oceans, salinity ranges only from 34.60 to 34.80 parts per thousand—a spread of just 200 parts per million. In the deep North Pacific the range is even narrower, about 20 ppm. To distinguish water masses by salinity, we therefore need measurement techniques accurate to roughly one part per million. Temperature, by contrast, varies by about 1°C and is far easier to measure. [Source: Robert Stewart, “Introduction to Physical Oceanography”, Texas A&M University, 2008]

ocean salinity in regards to depth
Formulating a practical, accurate definition of salinity has been challenging, and many versions have been used. The original definition—“the total amount of dissolved material in grams in one kilogram of seawater”—proved impractical because dissolved substances are nearly impossible to quantify directly. Volatile materials like gases are difficult to measure, and evaporating seawater to dryness is unreliable because chlorides are lost in the final stages.
To address these issues, the International Council for the Exploration of the Sea established a commission in 1889. Its 1902 definition described salinity as the “total amount of solid material in grams in one kilogram of seawater after converting all carbonates to oxides, replacing bromine and iodine with chlorine, and completely oxidizing all organic matter.” This was more workable but still too cumbersome for routine use.
By the 1960s, oceanographers began using conductivity meters to estimate salinity. Conductivity—the ability of a material to conduct electricity—can be measured precisely, and by the early 1970s instruments were available that could profile conductivity throughout the water column from research vessels.
Because the relative proportions of the major ions in seawater remain nearly constant across the open ocean (except in very fresh, estuarine waters), conductivity provides an extremely accurate proxy for salinity. The conductivity–salinity relationship has an accuracy of about ±0.003 in salinity; remaining small errors arise from constituents such as dissolved silica (SiO₂), which slightly affect density but not conductivity. This stability underpins the reliability of the long-standing relationships among chlorinity, salinity, and density, and therefore the accuracy of all density-based interpretations drawn from chemical or conductivity measurements.
Temperature and salinity (the amount of salt in something) are key to understanding the ocean and how it functions. See Temperature Under PHYSICS OF THE OCEAN
Why is the Ocean Salty?
Salt in the ocean comes from two sources: runoff from the land and openings in the seafloor. Rocks on land are the major source of salts dissolved in seawater. Rainwater that falls on land is slightly acidic, so it erodes rocks. This releases ions that are carried away to streams and rivers that eventually feed into the ocean. Many of the dissolved ions are used by organisms in the ocean and are removed from the water. Others are not removed, so their concentrations increase over time.
Another source of salts in the ocean is hydrothermal fluids, which come from vents in the seafloor. Ocean water seeps into cracks in the seafloor and is heated by magma from the Earth’s core. The heat causes a series of chemical reactions. The water tends to lose oxygen, magnesium, and sulfates, and pick up metals such as iron, zinc, and copper from surrounding rocks. The heated water is released through vents in the seafloor, carrying the metals with it. Some ocean salts come from underwater volcanic eruptions, which directly release minerals into the ocean.
Salt domes also contribute to the ocean's saltiness. These domes, vast deposits of salt that form over geological timescales, are found underground and undersea around the world. They are common across the continental shelf of the northwestern Gulf of Mexico.
Two of the most prevalent ions in seawater are chloride and sodium. Together, they make up around 85 percent of all dissolved ions in the ocean. Magnesium and sulfate make up another 10 percent of the total. Other ions are found in very small concentrations. The concentration of salt in seawater (salinity) varies with temperature, evaporation, and precipitation. Salinity is generally low at the equator and at the poles, and high at mid-latitudes. The average salinity is about 35 parts per thousand. Stated in another way, about 3.5 percent of the weight of seawater comes from the dissolved salts.
In the beginning, the primeval seas were probably only slightly salty. But over time, as rain fell to the Earth and ran over the land, breaking up rocks and transporting their minerals to the ocean, the ocean has become saltier. Rain replenishes freshwater in rivers and streams, so they don’t taste salty. However, the water in the ocean collects all of the salt and minerals from all of the rivers that flow into it. It is estimated that the rivers and streams flowing from the United States alone discharge 225 million tons of dissolved solids and 513 million tons of suspended sediment annually to the ocean. Throughout the world, rivers carry an estimated four billion tons of dissolved salts to the ocean annually. About the same tonnage of salt from ocean water probably is deposited as sediment on the ocean bottom and thus, yearly gains may offset yearly losses. In other words, the ocean today probably has a balanced salt input and output (and so the ocean is no longer getting saltier).
Conductivity and Forchhammer's Principle
Conductivity is a measure of how well a solution or material conducts electricity, calculated as the ratio of the current density in the material or solution to the electric field which causes the flow of current. Conductivity is directly related to salinity. [Source: NOAA]
Forchhammer's Principle refers to the chemical composition of ocean water. In 1865, the Danish geologist and mineralogist Johan Georg Forchhammer, with the help of naval and civilian collaborators, collected numerous samples of seawater from the Northern Atlantic and the Arctic Ocean. He wanted to determine why the salinity (or "saltiness") of seawater varies in different areas of the ocean.
Forchhammer put the samples through a detailed series of chemical analyses and found that the proportions of the major salts in seawater stay about the same everywhere. This constant ratio is known as Forchhammer's Principle, or the Principle of Constant Proportions. In addition to this principle, Forchhammer is credited with defining the term salinity to mean the concentration of major salts in seawater.
Forchhammer's discovery helped scientists understand that salinity levels in seawater vary due to the addition or removal of fresh water, rather than differing amounts of salt minerals in the water. The principle is still applied today in marine research, and provides a simple way to estimate salinity and trace the mixing of water masses in the global ocean.
CTD stands for conductivity, temperature, and depth, and refers to a package of electronic instruments that measure these properties. The primary function of CTD devise is to detect how the conductivity and temperature of the water column changes relative to depth.
Oxygen Production from the Ocean
Scientists estimate that 50 to 80 percent of the oxygen production on Earth comes from the ocean. The majority of this production is from oceanic plankton — drifting plants, algae, and some bacteria that can photosynthesize. The surface layer of the ocean is teeming with photosynthetic plankton. Though they're invisible to the naked eye, they produce more oxygen than the largest redwoods. One particular species, Prochlorococcus, is the smallest photosynthetic organism on Earth. But this little bacteria produces up to 20 percent of the oxygen in our entire biosphere. That’s a higher percentage than all of the tropical rainforests on land combined. [Source: NOAA]
Calculating the exact percentage of oxygen produced in the ocean is difficult because the amounts are constantly changing. Scientists can use satellite imagery to track photosynthesizing plankton and estimate the amount of photosynthesis occurring in the ocean, but satellite imagery cannot tell the whole story. The amount of plankton changes seasonally and in response to changes in the water’s nutrient load, temperature, and other factors. Studies have shown that the amount of oxygen in specific locations varies with time of day and with the tides.
It’s important to remember that although the ocean produces at least 50 percent of the oxygen on Earth, roughly the same amount is consumed by marine life. Like animals on land, marine animals use oxygen to breathe, and both plants and animals use oxygen for cellular respiration. Oxygen is also consumed when dead plants and animals decay in the ocean.
This is particularly problematic when algal blooms die and the decomposition process uses oxygen faster than it can be replenished. This can create areas of extremely low oxygen concentrations, or hypoxia. These areas are often called dead zones, because the oxygen levels are too low to support most marine life. NOAA’s National Centers for Coastal Ocean Science conducts extensive research and forecasting on algal blooms and hypoxia to lessen the harm done to the ocean ecosystem and human environment.
“Dark Oxygen” on the Seafloor
Scientists working in the Clarion-Clipperton Zone (CCZ) of the Pacific Ocean reported a startling finding: potato-sized polymetallic nodules on the seafloor—rich in cobalt, nickel, copper, and manganese—appear to generate electric voltages comparable to AA batteries. In experiments conducted with benthic chambers, oxygen levels unexpectedly increased in total darkness, leading the team, led by Andrew Sweetman of the Scottish Association for Marine Science, to propose that these nodules may split seawater into hydrogen and oxygen through natural electrolysis—producing what they called “dark oxygen.” [Source: AFP, March 17, 2025; July 23, 2024]
If true, the discovery could challenge long-held assumptions that oxygen on early Earth arose solely from photosynthetic microbes about 2.7–3 billion years ago. It might also expand ideas about where life could originate, both on Earth and on ocean-covered worlds such as Europa or Enceladus. Some environmental groups seized on the finding as further evidence that the deep-sea ecosystem is poorly understood and too fragile to withstand industrial mining.
But the claim has sharply divided the scientific community. Since its publication in Nature Geoscience, at least five academic papers have disputed the result, arguing that the observed oxygen may reflect contamination, trapped air bubbles, or flawed instrumentation. Critics—including researchers at GEOMAR and Ifremer—question whether nodules tens of millions of years old could still sustain the required electrical charge, and a major funder of the fieldwork, the mining firm The Metals Company, dismissed the study as methodologically unsound.
Sweetman maintains that scientific scrutiny is normal and is preparing a formal response. For now, the existence of “dark oxygen” remains unproven—an intriguing possibility that has ignited debate over Earth’s early oxygenation, deep-ocean chemistry, and the environmental risks of mining the abyss.
Carbon Cycle and Oceans
The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the Earth and then back into the atmosphere. The carbon cycle is nature's way of reusing carbon atoms, which travel from the atmosphere into organisms in the Earth and then back into the atmosphere over and over again. Carbon is the foundation for all life on Earth. Carbon is required to form complex molecules like proteins and DNA. This element is also found in our atmosphere in the form of carbon dioxide (CO2). Carbon helps to regulate the Earth’s temperature, makes all life possible, is a key ingredient in the food that sustains us, and provides a major source of the energy to fuel our global economy. [Source: NOAA]
Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. All of the carbon we currently have on Earth is the same amount we have always had. When new life is formed, carbon forms key molecules like protein and DNA. Where the carbon is located — in the atmosphere or on Earth — is constantly in flux. In the our atmosphere it is mostly in the form of carbon dioxide.
Most carbon is stored in rocks and sediments, while the rest is stored in the ocean, atmosphere, and living organisms. These are the reservoirs, or sinks, through which carbon cycles. Carbon is released back into the atmosphere when organisms die, volcanoes erupt, fires blaze, fossil fuels are burned, and through a variety of other mechanisms.
In the case of the ocean, carbon is continually exchanged between the ocean’s surface waters and the atmosphere, or is stored for long periods of time in the ocean depths. The ocean is a giant carbon sink that absorbs carbon. Marine organisms from marsh plants to fish, from seaweed to birds, also produce carbon through living and dying. Over millions of years, dead organisms can become fossil fuels. When humans burn these fuels for energy, vast amounts of carbon dioxide are released back into the atmosphere. This excess carbon dioxide changes our climate — increasing global temperatures, causing ocean acidification, and disrupting the planet’s ecosystems.
Human activities have a tremendous impact on this cycle. Burning fossil fuels, changing land use, and using limestone to make concrete all transfer massive quantities of carbon into the atmosphere. As a result, the amount of carbon dioxide in the atmosphere is rapidly rising — it is now greater than at any time in the last 3.6 million years.

Ocean carbon cycle and diatom carbon dioxide concentration mechanisms: Schematic representation of the ocean carbon cycle depicting the role of marine diatoms in the biological carbon pump. The anthropogenic CO2 emission to the atmosphere (mainly generated by fossil fuel burning and deforestation) is nearly 11 Gigaton carbon (GtC) per year, of which almost 2.5 GtC is taken up by the surface ocean. In surface seawater (pH 8.1–8.4), bicarbonate (HCO3–) and carbonate ions (CO32–) constitute nearly 90 and <10% of dissolved inorganic carbon (DIC) respectively, while dissolved CO2 (CO2 aqueous) contributes <1%. Despite this low level of CO2 in the ocean and its slow diffusion rate in water, diatoms fix 10–20 GtC annually via photosynthesis thanks to their carbon dioxide concentration mechanisms (CCMs), allowing them to sustain food chains. In addition, 0.1–1% of this organic material produced in the euphotic layer sinks down as particles, thus transferring the surface carbon toward the deep ocean and sequestering atmospheric CO2 for thousands of years or longer. The remaining organic matter is remineralized through respiration. Thus, diatoms are one of the main players in this biological carbon pump, which is arguably the most important biological mechanism in the Earth System allowing CO2 to be removed from the carbon cycle for very long period.
Blue Carbon
Blue carbon is the term for carbon captured by the world's ocean and coastal ecosystems. Sea grasses, mangroves, salt marshes, and other systems along our coast are very efficient in storing CO2. These areas also absorb and store carbon at a much faster rate than other areas, such as forests, and can continue to do so for millions of years. When these systems are damaged or disrupted by human activity, an enormous amount of carbon is emitted back into the atmosphere, contributing to climate change. [Source: NOAA]
Ocean and coasts provide a natural way of reducing the impact of greenhouse gases on our atmosphere, through sequestration (or taking in) of carbon. Sea grasses, mangroves, and salt marshes along our coast "capture and hold" carbon, acting as something called a carbon sink. These coastal systems, though much smaller in size than the planet's forests, sequester this carbon at a much faster rate, and can continue to do so for millions of years. Most of the carbon taken up by these ecosystems is stored below ground where we can't see it, but it is still there. The carbon found in coastal soil is often thousands of years old!
The bigger picture of blue carbon is one of coastal habitat conservation. When these systems are damaged, an enormous amount of carbon is emitted back into the atmosphere, where it can then contribute to climate change. So protecting and restoring coastal habitats is a good way to reduce climate change. When we protect the carbon in coastal systems, we protect healthy coastal environments that provide many other benefits to people, such as recreational opportunities, storm protection, and nursery habitat for commercial and recreational fisheries.
Ocean Acidification
Ocean acidification refers to a reduction in the pH of the ocean over an extended period of time, caused primarily by uptake of carbon dioxide (CO2) from the atmosphere. For more than 200 years, or since the industrial revolution, the concentration of carbon dioxide (CO2) in the atmosphere has increased due to the burning of fossil fuels and land use change. The ocean absorbs about 30 percent of the CO2 that is released in the atmosphere, and as levels of atmospheric CO2 increase, so do the levels in the ocean. [Source: National Oceanic and Atmospheric Administration (NOAA)]
In some places scientists have observed rises in acidity of 30 percent and predict 100 to 150 percent increases by 2100. The mixture of carbon dioxide and seawater creates carbonic acid, the weak acid in carbonated drinks. The increased acidity reduces the abundance of carbonate ions and other chemicals necessary to form calcium carbonate used make sea shells and coral skeletons. To get an idea what acid can due to shells remember back to high school chemistry classes when acid was added to calcium carbonate, causing it to fizz.
Ocean acidification is affecting the entire world’s oceans, including coastal estuaries and waterways. Many economies are dependent on fish and shellfish and people worldwide rely on food from the ocean as their primary source of protein. The acidification that has occurred so far is probably irreversible. Although in theory it's possible to add chemicals to the sea to counter the effects of the extra CO2, as a practical matter, the volumes involved would be staggering; it would take at least two tons of lime, for example, to offset a single ton of carbon dioxide, and the world now emits more than 30 billion tons of CO2 each year. Meanwhile, natural processes that could counter acidification — such as the weathering of rocks on land — operate far too slowly to make a difference on a human time-scale. Even if CO2 emissions were somehow to cease today, it would take tens of thousands of years for ocean chemistry to return to its pre-industrial condition.
See Separate Article OCEAN ACIDIFICATION factsanddetails.com
Hydrogen from Seawater: A Possible Clean Energy Source
As well all know hydrogen is component of H2O (water). It is the simplest atom and element, with a single positively charged proton and a single negatively charged electron, and hold great promise as cheap, renewable energy source, Hydrogen can be produced splitting water atoms and only produces water when it is burned. Use of its is expected to increase as the world uses alternatives to fossil fuels.
In February 2023, Researchers at the University of Adelaide said that they made clean hydrogen fuel from seawater without pre-treatment.“We have split natural seawater into oxygen and hydrogen with nearly 100 per cent efficiency, to produce green hydrogen by electrolysis, using a non-precious and cheap catalyst in a commercial electrolyser,” Professor Shizhang Qiao, the team’s co-leader, said. Seawater typically needs to be purified before electrolysis splits it into hydrogen and oxygen. The team says its results, using cobalt oxide with chromium oxide on its surface as the catalyst, had similar performance to a standard process of applying platinum and iridium catalysts to highly purified and deionized water. [Source: Will Shanklin, Engadget, February 4, 2023]

Ocean acidity: Aragonite saturation state is commonly used to track ocean acidification because it is a measure of carbonate ion concentration. When aragonite saturation state falls below 3, these organisms become stressed, and when saturation state is less than 1, shells and other aragonite structures begin to dissolve.
Endgadget reported: The findings could eventually provide cheaper green energy production to coastal areas. Compared to freshwater, seawater is an abundant resource, and the ability to extract hydrogen fuel from seawater without pre-treatment could save money. However, even if successfully scaled, it would likely only be practical for coastal communities with plenty of seawater. The team’s next step is to scale the system with a larger electrolyzer. Then, although it’s still early in development, the researchers hope to eventually apply the findings to commercial hydrogen production for fuel cells and ammonia synthesis. Co-lead Yao Zheng summarized, “Our work provides a solution to directly utilise seawater without pre-treatment systems and alkali addition, which shows similar performance as that of existing metal-based mature pure water electrolyser.”
Image Sources: Wikimedia Commons; YouTube, NOAA
Text Sources: National Oceanic and Atmospheric Administration (NOAA) noaa.gov; “Introduction to Physical Oceanography” by Robert Stewart , Texas A&M University, 2008 uv.es/hegigui/Kasper ; Wikipedia, National Geographic, Live Science, BBC, Smithsonian, New York Times, Washington Post, Los Angeles Times, The New Yorker, Reuters, Associated Press, Lonely Planet Guides and various books and other publications.
Last Updated November 2025



